As industry leaders in plastic surgery research and innovation, our board-certified plastic surgeons are at the forefront of advances shaping the future of aesthetic medicine. Their involvement in the development and FDA approval of Motiva breast implants once again highlights The Wall Center’s reputation as one of the most respected plastic surgery practices in the world.
Bridging Science & Patient Care: Our Surgeons’ Role in Motiva FDA Approval
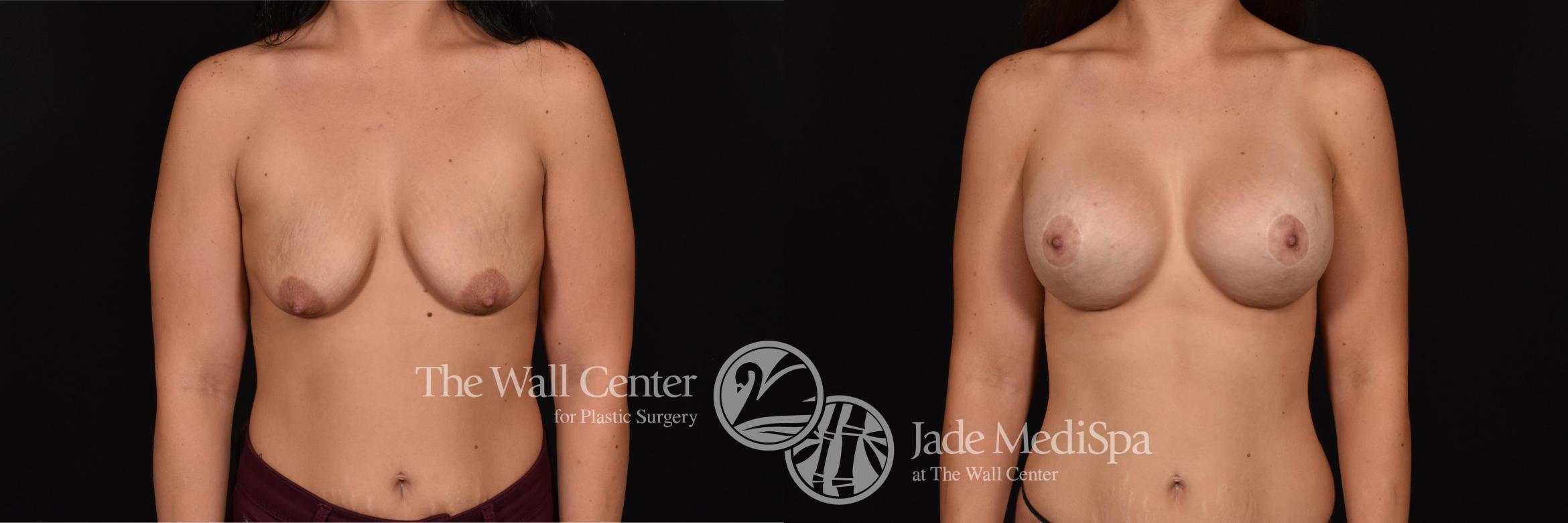
The Wall Center was at the center of research for the Motiva silicone gel breast implants. The FDA approved the breast implants in September 2024, making them the first new implants to earn FDA approval in more than 10 years. Because of their involvement in clinical research trials, our surgeons have been using Motiva implants for several years and are among the first to offer them to patients.
Dr. Wall Jr. and Dr. Holly Casey Wall are also part of the instructional team that will be teaching other plastic surgeons about the implants’ unique features.
What Are Motiva Breast Implants?
There are several types of Motiva implants for breast augmentation surgery. All are filled with 6th-generation silicone gel technology and available in a variety of implant sizes and dimensions.
- Motiva Ergonomix breast implants are filled with soft ProgressiveGel Ultima®. They look round when a patient lies down, but they take on an anatomical teardrop shape with some added fullness in the upper part of the breast when the patient stands.
- Motiva Round implants provide high projection and upper-pole fullness. They contain a silicone gel called ProgressiveGel PLUS, which gives them a firmer feel.
Patient and physician satisfaction with the results of Motiva breast implants exceeded 97% during the clinical trials.
How Safe Are Motiva Breast Implants?
Clinical data compiled as part of a 3-year study showed the implants are extremely safe and effective, with a very low risk of complications associated with the implants. For first-time breast augmentation patients, the rate of capsular contracture—the most common complication following breast augmentation—was less than 1%. There were also no reports of either BIA-ALCL (breast implant-associated anaplastic large cell lymphoma) or breast implant-associated squamous cell carcinoma (BIA-SCC) after 3 years.
A Legacy of Innovation
Ongoing research and development are an important part of our practice and are among the reasons our surgeons are highly respected by peers and patients alike. Advances pioneered by The Wall Center include SAFELipo® and expansion vibration lipofilling, liposuction, and fat grafting techniques. These techniques, developed by Dr. Simeon Wall Jr., are used by plastic surgeons throughout the U.S. and internationally.
As recognized leaders in breast enhancement surgery, Dr. Wall Jr., Dr. Holly Casey Wall, and Dr. Simeon Wall Sr. have collaborated with the country’s top breast implant manufacturers. This includes earning membership in the Mentor® LEAD (Leadership, Experience, and Development) breast augmentation program.
The Wall Center is also among only a handful of practices in the U.S. to earn Black Diamond status from Allergan, the maker of Natrelle® breast implants. Only practices with a distinguished track record of innovation, excellence, and the highest level of patient care achieve this recognition.
Get Started With a Consultation
Women considering breast augmentation travel to our Shreveport, LA, practice from throughout the nation and other countries to have one of our expert breast enhancement surgeons perform their procedure. Our surgeons help patients choose the best breast implants to achieve their aesthetic goals and suit their lifestyles. We’re excited to add FDA-approved Motiva implants to our choices. You can request a consultation at The Wall Center using the online form or by calling (318) 795-0801 to schedule an appointment.












Leave a Reply